Valbiotis has created a proprietary platform for its product development: Valbiotis R&D. This integrated Research and Development platform has been specifically designed for research on plants and applications in the field of metabolic and cardiovascular diseases. This cross-disciplinary entity brings together expert teams and infrastructures dedicated to plant-based chemistry, preclinical research on metabolism, and clinical development. It manages its entire product development chain from the discovery of active substances to the launch of clinical trials on humans, and complies with the highest quality standards, which contributed to Valbiotis achieving ISO 9001 certification.
Valbiotis R&D was designed to increase the creation of value over the long-term and to ensure the sustainable autonomy of the company.
Key figures
Team: 75% of the workforce at Valbiotis is assigned to Research and Development
Patents: 3 patent families filed by Valbiotis since 2014
Infrastructures: 2,600 m², of which 1,200 m² are dedicated to Discovery and Preclinical Research
Publications: 44 papers selected by international scientific and medical congresses since June 2016, and 10 scientific papers published by international journals since 2021.
R&D centers
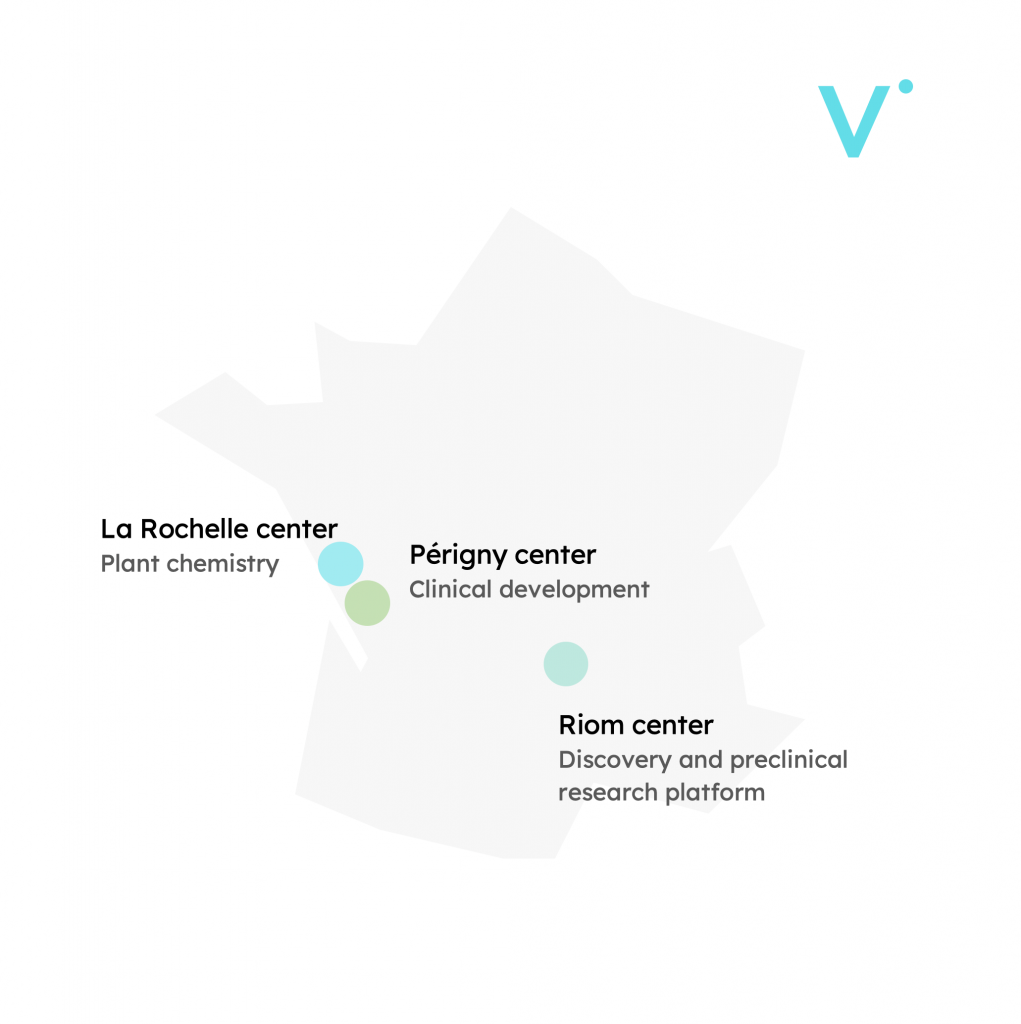
+ a subsidiary in Quebec at Nutrition and Functional Food Institute (INAF) from Laval University.