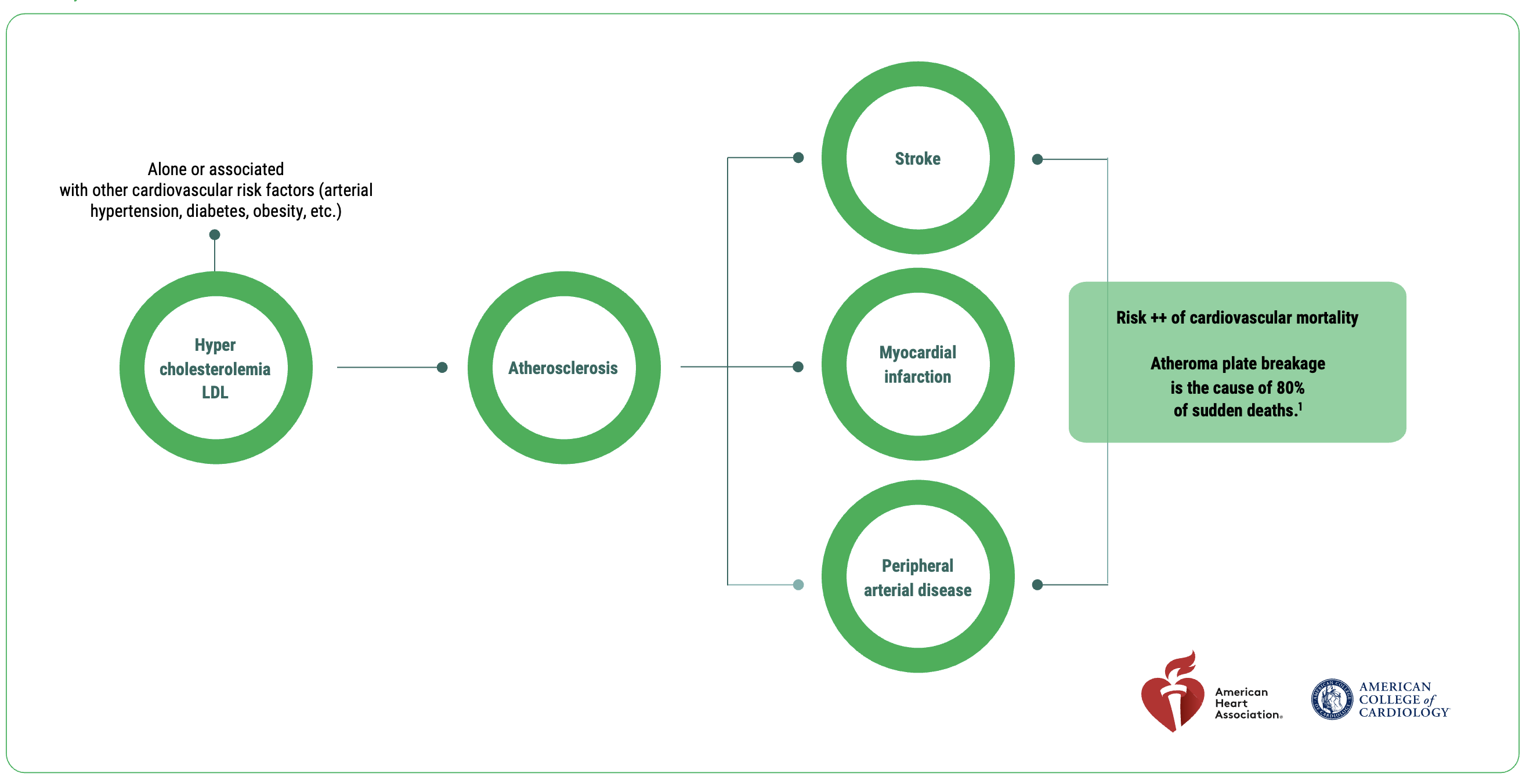
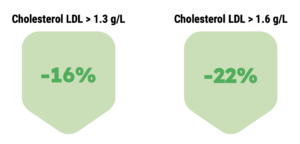
Lipidrive® (formerly TOTUM•070) is a unique patented combination of plant extracts, clinically proven for patients with untreated, mild to moderate LDL hypercholesterolemia, associated with a moderate overall cardiovascular risk.
- 15 communications in international scientific congresses since 2021: American Heart Association (AHA), European Society of Cardiology (ESC), European Atherosclerosis Society (EAS).
- 3 scientific publications in the international peer-reviewed: Nutrients and Frontiers in Cardiovascular Medicine.